Discovering New Mechanisms to Solve Significant Medical Challenges
For decades, the gut had been under-recognized as a viable site for new therapeutic points of intervention for treating disease. Using our discovery model, which recreates environments within certain areas of the gastrointestinal tract and kidney, Ardelyx scientists have been able to elucidate new and previously unexploited ion transport mechanisms. Focusing on these mechanisms, we are creating targeted, small molecule drugs that are orally active but with limited systemic absorption, aiming to develop potent and efficacious therapies that minimize the side effects and drug-drug interactions frequently encountered with traditional, systemically absorbed medicines.
Our novel biological insights have enabled us to develop a pipeline of drug candidates that specifically target these mechanisms, with the potential to benefit large populations of underserved patients.
Our lead candidate tenapanor,
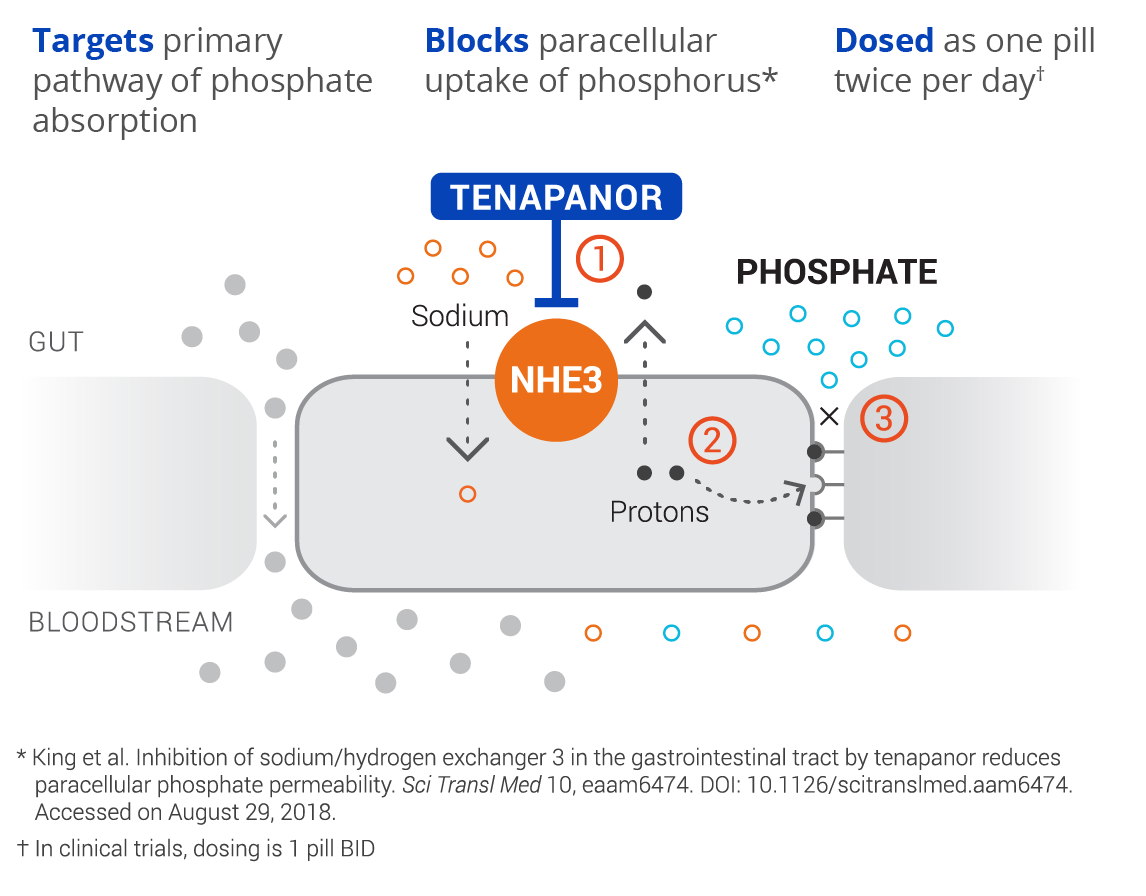
discovered and developed by Ardelyx, is a first-in-class, targeted therapy for the treatment of hyperphosphatemia in patients with chronic kidney disease (CKD) on dialysis. Tenapanor has a unique mechanism of action and acts locally in the gut to inhibit the sodium/hydrogen exchanger 3 (NHE3). This results in a conformational change of the epithelial cell junctions, reducing permeability specific to phosphate, resulting in decreased phosphate absorption through the paracellular pathway, the primary pathway of dietary phosphate absorption.
Following successful clinical development, we have now filed for U.S. regulatory approval of tenapanor for the control of serum phosphorus in adult patients with CKD on dialysis. If approved, tenapanor would provide a novel approach for the treatment of hyperphosphatemia (elevated serum phosphorus), a major co-morbidity factor in people with CKD and a significant unmet medical need.
Based on our successes to date in translating our scientific insights into promising targeted therapies, we are continuing our efforts and applying our expertise to uncover additional breakthroughs to develop first-in-class therapeutics to benefit patients.
Our discovery platform has also enabled the systematic study of the biological mechanisms of potassium secretion and has led us to the discovery of our lead candidate in the RDX013 program that pharmacologically targets potassium secretion through the lumen of the gut, thus lowering levels of serum potassium. High serum potassium, a condition called hyperkalemia, is a common problem in patients with kidney and heart disease and can cause a significantly increased risk of death because of the potential for heart conductance issues
What is CKD?
Chronic kidney disease (CKD) is the progressive deterioration of kidney function that can occur over time. The kidneys’ main job is to filter excess water and waste out of blood via urine. To keep the body working properly, the kidneys balance salts and minerals—such as calcium, phosphorus, sodium, and potassium—that circulate in the blood. Kidneys also make hormones that help control blood pressure, make red blood cells, and keep bones strong.
If deterioration of the kidneys continues, the disease will likely cause significant cardiovascular morbidity, and can progress to kidney failure.
Current management of kidney failure includes hemodialysis and peritoneal dialysis as a means to filter toxins from the blood once the kidneys have failed. Without dialysis or transplant, kidney failure results in the accumulation of waste products that may ultimately cause death. Hemodialysis, the most common form of dialysis, generally requires a patient to visit a dialysis center at least three times per week for a minimum three-hour session, significantly impacting quality of life.
There are currently estimated to be more than 35 million people in the U.S. suffering from chronic kidney disease, more than 550,000 of whom are treated with dialysis.
What is Hyperphosphatemia?
Hyperphosphatemia, a nearly universal condition among patients with CKD on dialysis, is an electrolyte disorder in which there is an elevated level of phosphate in the blood. Hyperphosphatemia is a major independent risk factor for cardiovascular morbidity and mortality in patients on dialysis. In fact, cardiovascular disease is the leading cause of death in this patient population and traditional risk factors alone do not explain the high rates of cardiovascular disease. Hyperphosphatemia has emerged as a predominant and modifiable risk factor for cardiovascular morbidity and mortality, and as such, effective management of serum phosphorus is critical for patients with CKD on dialysis.
While dialysis is the basis for homeostatic electrolyte management, dialysis regimens are unable to successfully remove excess phosphate in order to achieve a neutral phosphate balance. As a result, approximately 80% of patients with CKD on dialysis require phosphate-lowering therapy on top of restrictive, low phosphorus diets.
Despite widespread treatment with currently available therapies, a significant proportion of patients are unable to achieve and maintain target phosphorus levels. While the 2017 KDIGO clinical practice guidelines recommend lowering elevated phosphate levels toward the normal range (2.5-4.5 mg/dL or 0.81-1.45mmol/L), due to the difficulties in managing phosphorus, most clinicians target phosphorus levels between 3.5-5.5 mg/dL (1.13-1.78 mmol/L), based on the 2003 KDOQI clinical practice guidelines. Even the less aggressive targets are often unachievable today with approximately 40% of patients having phosphorus levels >5.5 mg/dL (1.78 mmol/L) in any given month, and approximately 80% of patients unable to consistently maintain phosphorus levels ≤5.5 mg/dL (1.78 mmol/L) over a 6-month period.
Currently available treatments all belong to the class of drugs referred to as phosphate binder therapies. Achieving and maintaining effective phosphate control with phosphate binders is extremely challenging. Phosphate binders act by binding dietary phosphorus in the gut. The binding mechanism requires frequent dosing and often, many large pills in order to bind enough phosphorus, making phosphate binders the largest contributor to excessive pill burden for patients on dialysis. Phosphate binders also tend to be associated with a number of GI side effects including nausea, vomiting, abdominal pain, diarrhea, and constipation.
Here at Ardelyx, we have discovered that the paracellular pathway is the primary mechanism by which dietary phosphorus is absorbed. By developing first-in-class therapeutics to specifically target and block phosphorus absorption through the paracellular pathway, we hope to address what continues to be a significant unmet medical need in patients on dialysis.
What is Hyperkalemia?
Hyperkalemia is a potentially life-threatening condition in which blood levels of potassium are elevated above normal. Potassium is a nutrient that is critical to the normal function of nerve and muscle cells, including those in the heart. Normal potassium blood levels are tightly balanced and maintained primarily by the kidneys. For people with chronic kidney disease (CKD), heart failure, and diabetes, and particularly those also taking highly beneficial renin-angiotensin-aldosterone system (RAAS) inhibitors, there is a greater risk of developing hyperkalemia due to side effects and the kidney’s limited ability to keep potassium in balance.
Because of the risk of hyperkalemia, several published guidelines have suggested that physicians should reduce and possibly discontinue RAAS inhibitors in order to manage the risk of hyperkalemia in CKD and heart failure patients. The alternative medications used to control hypertension, including diuretics and calcium channel blockers, are less effective than RAAS inhibitors, particularly in patients with failing kidneys and severe hypertension.
According to the 2015 publication Market Dynamix: Hyperkalemia, released by Spherix Global Insights, U.S. cardiologists reported that of the patients who would benefit from RAAS inhibition, up to 38% of patients with heart failure and up to 55% of patients with both heart failure and CKD are being administered a sub-optimal dose or none at all. Nephrologists reported that at least one-third of patients who would benefit from RAAS inhibition receive a sub-optimal dose or none at all. We believe there is clearly a strong medical need for new medications that control hyperkalemia in order to allow for optimal use of RAAS inhibitors to control hypertension in these patient populations.
It is estimated that 2.1 million people in the U.S. with CKD and/or heart failure have hyperkalemia, which remains an emerging and unaddressed market with today’s treatments.
What is IBSRELA®?
IBSRELA® (tenapanor) 50 mg tablets is a prescription medicine used in adults to treat Irritable Bowel Syndrome with Constipation (IBS-C). It is not known if IBSRELA is safe and effective in children less than 18 years of age.
Irritable bowel syndrome with constipation (IBS-C) is a gastrointestinal disorder characterized by significant abdominal pain and constipation. IBS-C significantly impacts the health and quality of life of affected patients. The cause of IBS-C is unknown, and there are currently no specific diagnostic tests or biomarkers for detection. Therefore, IBS-C is diagnosed by symptoms and by eliminating other disorders. It is estimated that there are ~11 million people in the U.S. with IBS-C.
IMPORTANT SAFETY INFORMATION
- Do not give IBSRELA to children who are less than 6 years of age. It may harm them.
- You should not give IBSRELA to patients 6 years to less than 18 years of age. It may harm them. IBSRELA can cause severe diarrhea and your child could get severe dehydration (loss of a large amount of body water and salt).
- Do not take IBSRELA if a doctor has told you that you have a bowel blockage (intestinal obstruction).
Before you take IBSRELA, tell your doctor:
- If you have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if IBSRELA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if IBSRELA passes into your breast milk. Talk with your doctor about the best way to feed your baby if you take IBSRELA®.
- Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Side Effects
- Diarrhea is the most common side effect of IBSRELA, and it can sometimes be severe. Stop taking IBSRELA and call your doctor if you develop severe diarrhea.
The other most common side effects of IBSRELA include:
- swelling, or a feeling of fullness or pressure in your abdomen (distension).
- gas (flatulence).
- dizziness
These are not all the possible side effects of IBSRELA. Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to www.fda.gov/medwatch or you can report side effects to Ardelyx at 1-844-427-7352.
Please also see Medication Guide within the full Prescribing Information.
What is IBS-C?
Irritable bowel syndrome with constipation (IBS-C) is a gastrointestinal disorder characterized by abdominal pain and constipation, that significantly impacts the health and quality of life of affected patients. IBS-C is a common condition with a multi-factorial pathophysiology. There are currently no specific tests or biomarkers for diagnosis of IBS-C. Therefore, IBS-C is diagnosed by symptoms and by eliminating other disorders.
It is estimated that there are ~11 million people in the U.S. with IBS-C.
Our First Discovery
Our unique discovery platform and deep understanding of the primary mechanism of sodium transport in the intestine resulted in our discovery and development of IBSRELA® (tenapanor), a first-in-class therapy that received approval in the U.S. for the treatment of adults with irritable bowel syndrome with constipation (IBS-C). We are pursuing strategic collaborations for IBSRELA for IBS-C in the U.S. and have established commercialization agreements with Fosun Pharma in China and Knight in Canada for this indication.
Scientific Publications
IBS-C
2018 10. World Congress of Gastroenterology at ACG 2018. An Open-label, Long-term Safety Trial of Tenapanor in Patients with Irritable Bowel Syndrome with Constipation (IBS-C): T3MPO-3.
2017 10. World Congress of Gastroenterology at ACG 2017. Tenapanor Reduces IBS Pain Through Inhibition of TRPV1-dependent Neuronal Hyperexcitability In Vivo.
2017 10. World Congress of Gastroenterology at ACG 2017. Efficacy and Safety of Tenapanor in Patients with Constipation-Predominant Irritable Bowel Syndrome: A 12-Week, Double-Blind, Placebo-Controlled, Randomized Phase 3 Trial.
2017 02. American Journal of Gastroenterology. Tenapanor Treatment of Patients With Constipation-Predominant Irritable Bowel Syndrome: A Phase 2, Randomized, Placebo-Controlled Efficacy and Safety Trial.
2016 10. American College of Gastroenterology Annual Meeting 2016. Effect of Tenapanor on Global Endpoints in Patients with IBS-C: Results from a 12-Week, Double-Blind, Placebo-Controlled, Randomized Phase 2b Trial.
2015 05. Digestive Disease Week 2015. Efficacy and Safety of Tenapanor in Patients with Constipation-predominant Irritable Bowel Syndrome: a 12-week, Double-blind, Placebo-controlled, Randomized Phase 2b Trial.
Hyperphosphatemia
2018. Nephrology Dialysis Transplant. The Effects of Tenapanor on Serum Fibroblast Growth Factor 23 in Patients Receiving Hemodialysis with Hyperphosphatemia.
2017 11. American Society of Nephrology Kidney Week 2017. Gastrointestinal Tolerability of Tenapanor to Treat Hyperphosphatemia in Patients on Hemodialysis.
2017 11. American Society of Nephrology Kidney Week 2017. Efficacy of Tenapanor to Treat Hyperphosphatemia in Patients on Hemodialysis.
2017. Journal of the American Society of Nephrology. Effect of Tenapanor on Serum Phosphate in Patients Receiving Hemodialysis.
2016 09. Clinical Pharmacology in Drug Development. Preclinical and Healthy Volunteer Studies of Potential Drug–Drug Interactions Between Tenapanor and Phosphate Binders.
2015 11. American Society of Nephrology Kidney Week 2015. A Phase 2 Study on the Effect of Tenapanor on Albuminuria in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease.
2015 11. American Society of Nephrology Kidney Week 2015. Tenapanor, a Gastrointestinal NHE3 Inhibitor, Reduces Serum Phosphate in Patients with Chronic Kidney Disease Stage 5D and Hyperphosphatemia.
2014 11. American Society of Nephrology Kidney Week 2014. Tenapanor, a Minimally Absorbed NHE3 Inhibitor, Reduces Dietary Phosphorus Absorption in Healthy Volunteers.
2014 11. American Society of Nephrology Kidney Week 2014. Tenapanor Inhibits Phosphorous Absorption and Protects Against Vascular Calcification in Nephrectomized Rats.
2012 05. 49th ERA-EDTA Congress. RDX5791, a Non-systemic NHE3 Inhibitor for the Treatment of Fluid and Sodium Overload, Shifts Sodium Excretion from Urine to Feces in Healthy Subjects.
General Clinical
2018 01. Clinical Drug Investigation. Pharmacodynamics, Safety, and Tolerability of the NHE3 Inhibitor Tenapanor: Two Trials in Healthy Volunteers.
2017 04. British Journal of Clinical Pharmacology. Tenapanor Administration and the Activity of the H+-coupled Transporter PepT1 in Healthy Volunteers.
2017 01. Clinical Pharmacology in Drug Development. Effect of Food Intake on the Pharmacodynamics of Tenapanor: A Phase 1 Study.
2017 01. Clinical Pharmacology in Drug Development. Effects of Tenapanor on Cytochrome P450-Mediated Drug-Drug Interactions.
2016 06. Clinical and Experimental Nephrology. A Phase 1 Study of the Safety, Tolerability, Pharmacodynamics, and Pharmacokinetics of Tenapanor in Healthy Japanese Volunteers.
Research
2018 06. Digestive Disease Week 2018. RDX023-2, a Minimally Systemic, Non-bile Acid FXR Agonist, Reduces Steatosis, Inflammation and Fibrosis in Three Mouse Models of NASH.
2018 06. Digestive Disease Week 2018. Inhibition of Gastrointestinal (GI) NHE3 Normalizes GI Transit in Models of Opioid-induced Constipation, Multiple Sclerosis and Cystic Fibrosis.
2018 06. Digestive Disease Week 2018. Tenapanor Attenuates Increased Macromolecule Permeability in Human Colon Monolayer Cultures Induced by Inflammatory Cytokines and Human Fecal Supernatants.
2015. Current Opinion – Nephrology Hypertension. Pharmacologic Inhibition of Intestinal Sodium Uptake: A Gut Centric Approach to Sodium Management.
2015 11. American Society of Nephrology Kidney Week 2015. Prophylactic and Therapeutic Tenapanor are Vascular Protective in a Rat Model of Chronic Kidney Disease.
2014 03. Science Translational Medicine. Intestinal Inhibition of the Na+/H+ Exchanger 3 Prevents Cardiorenal Damage in Rats and Inhibits Na+ Uptake in Humans.
2014. Journal of the American Society of Nephrology. Gastrointestinal Inhibition of Sodium-Hydrogen Exchanger 3 Reduces Phosphorus Absorption and Protects against Vascular Calcification in CKD.


